DETECT EARLY,
SAVE LIVES GLOBALLY
Introducing,
mirascope®
A handheld breast cancer screening device that detects abnormal tissue stiffness, allowing for early triage or referrals
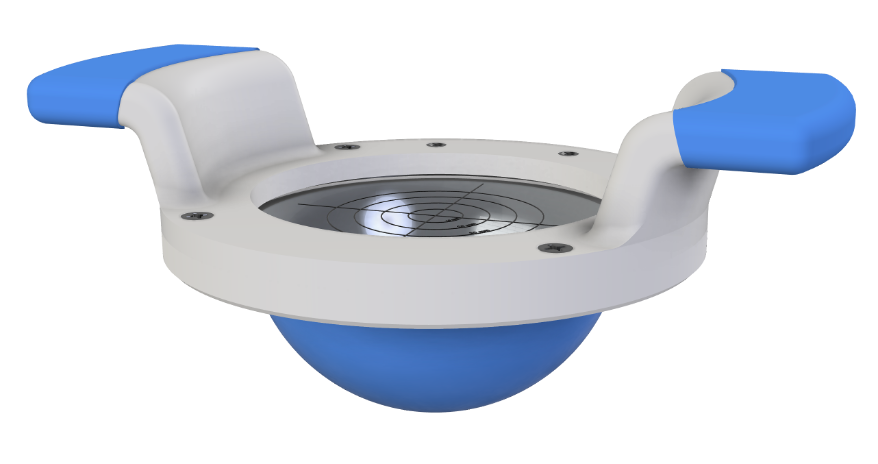
mirascope®
Handheld Screening Device
Mirascope is a research-stage device concept and is not cleared, approved, or authorized by the U.S. FDA.
It is not available for sale, clinical use, or investigational use.
Any descriptions reflect early-stage development and ongoing research and do not represent established safety or effectiveness. We are preparing for FDA engagement through the Q-Submission process.

How It Works
Simple, effective technology that detects tissue stiffness variations
Apply Pressure
Healthcare worker uses the handles to press mirascope® against the patient's breast
Detect Stiffness
Cancer tissue is 13x stiffer than surrounding tissue. Elastomeric lens deforms with the presence of a growth and creates a topographic highlight of the denser tissue
Measure & Refer
Measuring growth rings to track growths, which should be immediately further examined
Note: Clinical management decisions should only be made on the basis of the clinical and diagnostic examination (e.g. ultrasound or mammography)
Current Status
Driving clinical validation and advancing regulatory milestones
Piloted in outskirts of Kumasi, Ghana with OKB Hope Foundation
Pending provisional patent application with U.S. Patent and Trademark Office
Planning medical trials in Africa in Q4 2025 and preparing FDA regulatory documentation